dUMP (ECMDB01409) (M2MDB000375)
| Record Information | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 2.0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Creation Date | 2012-05-31 13:52:00 -0600 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 2015-09-13 12:56:11 -0600 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Secondary Accession Numbers |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
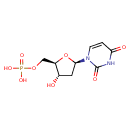
| Name: | dUMP | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description | dUMP is formed by the reduction of ribonucleotides to deoxyribonucleotides by ribonucleoside diphosphate reductase [EC 1.17.4.1]. dUMP by the action of by thymidylate synthetase [EC 2.1.1.45] produces dTMP (5,10-Methylene-5,6,7,8-tetrahydrofolate is a cofactor for the reaction). The nuclear form of uracil-DNA glycosylase (UNG2), that its major role is to remove misincorporated dUMP residues (cells deficient in removal of misincorporated dUMP accumulate uracil residues). (PMID 11554311) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula: | C9H13N2O8P | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Weight: | Average: 308.1819 Monoisotopic: 308.040951914 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key: | JSRLJPSBLDHEIO-SHYZEUOFSA-N | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI: | InChI=1S/C9H13N2O8P/c12-5-3-8(11-2-1-7(13)10-9(11)14)19-6(5)4-18-20(15,16)17/h1-2,5-6,8,12H,3-4H2,(H,10,13,14)(H2,15,16,17)/t5-,6+,8+/m0/s1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number: | 964-26-1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name: | {[(2R,3S,5R)-5-(2,4-dioxo-1,2,3,4-tetrahydropyrimidin-1-yl)-3-hydroxyoxolan-2-yl]methoxy}phosphonic acid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional IUPAC Name: | 2'-deoxyuridylic acid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES: | O[C@H]1C[C@@H](O[C@@H]1COP(O)(O)=O)N1C=CC(=O)NC1=O | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description | belongs to the class of organic compounds known as pyrimidine 2'-deoxyribonucleoside monophosphates. These are pyrimidine nucleotides with a monophosphate group linked to the ribose moiety lacking a hydroxyl group at position 2. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Organic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Nucleosides, nucleotides, and analogues | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Pyrimidine nucleotides | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Pyrimidine deoxyribonucleotides | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | Pyrimidine 2'-deoxyribonucleoside monophosphates | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Aromatic heteromonocyclic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State: | Solid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Charge: | -2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations: | Cytoplasm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reactions: | dUMP + Water > Deoxyuridine + Phosphate Deoxyuridine triphosphate + Water <> dUMP + Hydrogen ion + Pyrophosphate Adenosine triphosphate + Deoxyuridine > ADP + dUMP + Hydrogen ion dUMP + 5,10-Methylene-THF <> Dihydrofolic acid + 5-Thymidylic acid Adenosine triphosphate + dUMP <> ADP + dUDP Adenosine triphosphate + Deoxyuridine <> ADP + dUMP Deoxyuridine triphosphate + Water <> dUMP + Pyrophosphate Deoxyuridine triphosphate + Water > Hydrogen ion + dUMP + Pyrophosphate dUMP + 5,10-Methylene-THF > 5-Thymidylic acid + Dihydrofolic acid Deoxyuridine triphosphate + Water > dUMP + Pyrophosphate Dihydrofolic acid + 5-Thymidylic acid + Dihydrofolic acid > 5,10-Methylene-THF + dUMP + 5,10-Methylene-THF dUMP + 5,10-methenyltetrahydrofolate mono-L-glutamate + 5,10-methenyltetrahydrofolate mono-L-glutamate > Dihydrofolic acid + 5-Thymidylic acid + Dihydrofolic acid Deoxyuridine triphosphate + Water > Phosphate + Hydrogen ion + dUMP dUMP + 5 5,10-Methylene-THF <> Dihydrofolic acid +5 5-Thymidylic acid Adenosine triphosphate + dUMP <> ADP + dUDP Deoxyuridine triphosphate + Water <> dUMP + Hydrogen ion + Pyrophosphate dUMP + 5 5,10-Methylene-THF <> Dihydrofolic acid +5 5-Thymidylic acid Deoxyuridine triphosphate + Water <> dUMP + Hydrogen ion + Pyrophosphate | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMPDB Pathways: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| KEGG Pathways: | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| EcoCyc Pathways: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Concentrations | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra: | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Material Safety Data Sheet (MSDS) | Download (PDF) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Links | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Enzymes
- General function:
- Involved in hydrolase activity
- Specific function:
- This enzyme is involved in nucleotide metabolism:it produces dUMP, the immediate precursor of thymidine nucleotides and it decreases the intracellular concentration of dUTP so that uracil cannot be incorporated into DNA
- Gene Name:
- dut
- Uniprot ID:
- P06968
- Molecular weight:
- 16155
Reactions
| dUTP + H(2)O = dUMP + diphosphate. |
- General function:
- Involved in hydrolase activity
- Specific function:
- Degradation of external UDP-glucose to uridine monophosphate and glucose-1-phosphate, which can then be used by the cell
- Gene Name:
- ushA
- Uniprot ID:
- P07024
- Molecular weight:
- 60824
Reactions
| UDP-sugar + H(2)O = UMP + alpha-D-aldose 1-phosphate. |
| A 5'-ribonucleotide + H(2)O = a ribonucleoside + phosphate. |
- General function:
- Involved in thymidylate kinase activity
- Specific function:
- Catalyzes the reversible phosphorylation of deoxythymidine monophosphate (dTMP) to deoxythymidine diphosphate (dTDP), using ATP as its preferred phosphoryl donor. Situated at the junction of both de novo and salvage pathways of deoxythymidine triphosphate (dTTP) synthesis, is essential for DNA synthesis and cellular growth
- Gene Name:
- tmk
- Uniprot ID:
- P0A720
- Molecular weight:
- 23783
Reactions
| ATP + dTMP = ADP + dTDP. |
- General function:
- Involved in hydrolase activity
- Specific function:
- Nucleotidase with a broad substrate specificity as it can dephosphorylate various ribo- and deoxyribonucleoside 5'- monophosphates and ribonucleoside 3'-monophosphates with highest affinity to 3'-AMP. Also hydrolyzes polyphosphate (exopolyphosphatase activity) with the preference for short-chain- length substrates (P20-25). Might be involved in the regulation of dNTP and NTP pools, and in the turnover of 3'-mononucleotides produced by numerous intracellular RNases (T1, T2, and F) during the degradation of various RNAs. Also plays a significant physiological role in stress-response and is required for the survival of E.coli in stationary growth phase
- Gene Name:
- surE
- Uniprot ID:
- P0A840
- Molecular weight:
- 26900
Reactions
| A 5'-ribonucleotide + H(2)O = a ribonucleoside + phosphate. |
| A 3'-ribonucleotide + H(2)O = a ribonucleoside + phosphate. |
| (Polyphosphate)(n) + H(2)O = (polyphosphate)(n-1) + phosphate. |
- General function:
- Involved in thymidylate synthase activity
- Specific function:
- Provides the sole de novo source of dTMP for DNA biosynthesis. This protein also binds to its mRNA thus repressing its own translation
- Gene Name:
- thyA
- Uniprot ID:
- P0A884
- Molecular weight:
- 30480
Reactions
| 5,10-methylenetetrahydrofolate + dUMP = dihydrofolate + dTMP. |
- General function:
- Involved in catalytic activity
- Specific function:
- Nucleotidase that shows high phosphatase activity toward three nucleoside 5'-monophosphates, UMP, dUMP, and dTMP, and very low activity against TDP, IMP, UDP, GMP, dGMP, AMP, dAMP, and 6- phosphogluconate. Is strictly specific to substrates with 5'- phosphates and shows no activity against nucleoside 2'- or 3'- monophosphates. Might be involved in the pyrimidine nucleotide substrate cycles
- Gene Name:
- yjjG
- Uniprot ID:
- P0A8Y1
- Molecular weight:
- 25300
Reactions
| A 5'-ribonucleotide + H(2)O = a ribonucleoside + phosphate. |
- General function:
- Involved in acid phosphatase activity
- Specific function:
- Dephosphorylates several organic phosphomonoesters and catalyzes the transfer of low-energy phosphate groups from phosphomonoesters to hydroxyl groups of various organic compounds. Preferentially acts on aryl phosphoesters. Might function as a broad-spectrum dephosphorylating enzyme able to scavenge both 3'- and 5'-nucleotides and also additional organic phosphomonoesters
- Gene Name:
- aphA
- Uniprot ID:
- P0AE22
- Molecular weight:
- 26103
Reactions
| A phosphate monoester + H(2)O = an alcohol + phosphate. |
- General function:
- Involved in nucleoside-triphosphate diphosphatase activity
- Specific function:
- Specific function unknown
- Gene Name:
- mazG
- Uniprot ID:
- P0AEY3
- Molecular weight:
- 30412
Reactions
| ATP + H(2)O = AMP + diphosphate. |
- General function:
- Involved in thymidine kinase activity
- Specific function:
- Phosphorylates both thymidine and deoxyuridine
- Gene Name:
- tdk
- Uniprot ID:
- P23331
- Molecular weight:
- 23456
Reactions
| ATP + thymidine = ADP + thymidine 5'-phosphate. |
- General function:
- Involved in hydrolase activity, acting on acid anhydrides, in phosphorus-containing anhydrides
- Specific function:
- Catalyzes the hydrolysis of nucleoside triphosphates, with a preference for pyrimidine deoxynucleoside triphosphates (dUTP, dTTP and dCTP)
- Gene Name:
- nudI
- Uniprot ID:
- P52006
- Molecular weight:
- 16371
Reactions
| Nucleoside triphosphate + H(2)O = nucleoside monophosphate + diphosphate. |
- General function:
- Involved in hydrolase activity
- Specific function:
- Hydrolyzes O6 atom-containing purine bases deoxyinosine triphosphate (dITP) and xanthosine triphosphate (XTP) as well as 2'-deoxy-N-6-hydroxylaminopurine triposphate (dHAPTP) to nucleotide monophosphate and pyrophosphate. Probably excludes non- standard purines from DNA precursor pool, preventing thus incorporation into DNA and avoiding chromosomal lesions
- Gene Name:
- rdgB
- Uniprot ID:
- P52061
- Molecular weight:
- 21039
Reactions
| A nucleoside triphosphate + H(2)O = a nucleotide + diphosphate. |
- General function:
- Involved in catalytic activity
- Specific function:
- Nucleotidase that shows strict specificity toward deoxyribonucleoside 5'-monophosphates and does not dephosphorylate 5'-ribonucleotides or ribonucleoside 3'-monophosphates. Might be involved in the regulation of all dNTP pools in E.coli
- Gene Name:
- yfbR
- Uniprot ID:
- P76491
- Molecular weight:
- 22708
Reactions
| A 5'-ribonucleotide + H(2)O = a ribonucleoside + phosphate. |
Transporters
- General function:
- Involved in transporter activity
- Specific function:
- Non-specific porin
- Gene Name:
- ompN
- Uniprot ID:
- P77747
- Molecular weight:
- 41220
- General function:
- Involved in transporter activity
- Specific function:
- Uptake of inorganic phosphate, phosphorylated compounds, and some other negatively charged solutes
- Gene Name:
- phoE
- Uniprot ID:
- P02932
- Molecular weight:
- 38922
- General function:
- Involved in transporter activity
- Specific function:
- OmpF is a porin that forms passive diffusion pores which allow small molecular weight hydrophilic materials across the outer membrane. It is also a receptor for the bacteriophage T2
- Gene Name:
- ompF
- Uniprot ID:
- P02931
- Molecular weight:
- 39333
- General function:
- Involved in transporter activity
- Specific function:
- Forms passive diffusion pores which allow small molecular weight hydrophilic materials across the outer membrane
- Gene Name:
- ompC
- Uniprot ID:
- P06996
- Molecular weight:
- 40368