| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2012-05-31 14:30:09 -0600 |
|---|
| Update Date | 2015-06-03 17:19:25 -0600 |
|---|
| Secondary Accession Numbers | |
|---|
| Identification |
|---|
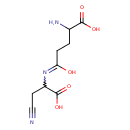
| Name: | gamma-Glutamyl-beta-cyanoalanine |
|---|
| Description | Gamma-glutamyl-beta-cyanoalanine belongs to the class of Alpha Amino Acids and Derivatives. These are amino acids in which the amino group is attached to the carbon atom immediately adjacent to the carboxylate group (alpha carbon). |
|---|
| Structure | |
|---|
| Synonyms: | - 2-amino-5-(1-Carboxy-2-cyanoethyl)amino-5-oxopentanoate
- 2-amino-5-(1-carboxy-2-cyanoethyl)amino-5-oxopentanoic acid
- g-Glutamyl-b-cyanoalanine
- γ-Glutamyl-β-cyanoalanine
|
|---|
| Chemical Formula: | C9H13N3O5 |
|---|
| Weight: | Average: 243.2166
Monoisotopic: 243.085520541 |
|---|
| InChI Key: | QUAADUAOVLZBJM-UHFFFAOYSA-N |
|---|
| InChI: | InChI=1S/C9H13N3O5/c10-4-3-6(9(16)17)12-7(13)2-1-5(11)8(14)15/h5-6H,1-3,11H2,(H,12,13)(H,14,15)(H,16,17) |
|---|
| CAS number: | Not Available |
|---|
| IUPAC Name: | 2-amino-4-[(1-carboxy-2-cyanoethyl)-C-hydroxycarbonimidoyl]butanoic acid |
|---|
| Traditional IUPAC Name: | gamma-glutamyl-β-cyanoalanine |
|---|
| SMILES: | NC(CCC(O)=NC(CC#N)C(O)=O)C(O)=O |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as dipeptides. These are organic compounds containing a sequence of exactly two alpha-amino acids joined by a peptide bond. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | Dipeptides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Alpha-dipeptide
- Gamma-glutamyl alpha-amino acid
- Glutamine or derivatives
- N-acyl-alpha-amino acid
- N-acyl-alpha amino acid or derivatives
- Alpha-amino acid
- Alpha-amino acid or derivatives
- Dicarboxylic acid or derivatives
- Fatty acyl
- Fatty acid
- Fatty amide
- N-acyl-amine
- Amino acid or derivatives
- Carboxamide group
- Amino acid
- Secondary carboxylic acid amide
- Carboxylic acid
- Nitrile
- Carbonitrile
- Primary aliphatic amine
- Organooxygen compound
- Primary amine
- Hydrocarbon derivative
- Organic oxide
- Carbonyl group
- Organopnictogen compound
- Amine
- Organonitrogen compound
- Organic nitrogen compound
- Organic oxygen compound
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Physical Properties |
|---|
| State: | Not Available |
|---|
| Charge: | -1 |
|---|
| Melting point: | Not Available |
|---|
| Experimental Properties: | |
|---|
| Predicted Properties | |
|---|
| Biological Properties |
|---|
| Cellular Locations: | Cytoplasm |
|---|
| Reactions: | |
|---|
| SMPDB Pathways: | |
|---|
| KEGG Pathways: | |
|---|
| EcoCyc Pathways: | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| Spectra |
|---|
| Spectra: | |
|---|
| References |
|---|
| References: | - Kanehisa, M., Goto, S., Sato, Y., Furumichi, M., Tanabe, M. (2012). "KEGG for integration and interpretation of large-scale molecular data sets." Nucleic Acids Res 40:D109-D114. Pubmed: 22080510
|
|---|
| Synthesis Reference: | Not Available |
|---|
| Material Safety Data Sheet (MSDS) | Not Available |
|---|
| Links |
|---|
| External Links: | | Resource | Link |
|---|
| CHEBI ID | Not Available | | HMDB ID | Not Available | | Pubchem Compound ID | 440768 | | Kegg ID | C05711 | | ChemSpider ID | 389637 | | Wikipedia ID | Not Available | | BioCyc ID | Not Available |
|
|---|